Selective Catalytic Reduction (SCR) is a term used in the air pollution control industry for the process of selectively targeting a pollutant to remove that pollutant from an exhaust stream. The most common pollutant that is controlled through a SCR process is nitrogen oxide (NOx). While there are many catalysts that can selectively target a number of compounds, the content of this blog will focus on SCR technologies for the reduction of NOx.
 The NOx reduction process in SCR technologies relies on three components:
The NOx reduction process in SCR technologies relies on three components:
NOx (NO + NO2) Being the Target Pollutant
NOx abatement technologies are very common and widely used across many industrial operations. NOx is a term for oxides of nitrogen, such as nitric oxide (NO) and nitrogen dioxide (NO2). NOx emissions are harmful to the environment because they contribute to acid rain, result in elevated fine particulate matter, and are pre-cursors of petrochemical smog (or ozone). Minimizing ground level ozone is the foundation of air pollution control for volatile organic compounds (VOC) and NOx.
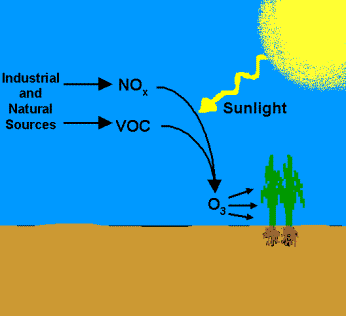
Visit this link to learn more about recent
EPA developments regarding ozone
There are many other forms of nitrogen oxides, and one that has gained particular attention recently is nitrous oxide (N2O), because it is a regulated Greenhouse Gas. NOx abatement systems are commonly used in large utility boilers that are used for electrical power generation, internal combustion engines, gas turbines, and mobile sources such as locomotives, ships, trucks, and automobiles.
A Catalyst Designed to Complete Certain Chemical Reactions
There are several types of catalyst that work in SCR processes. Below is a simplified example of the reaction that takes place in a SCR process:
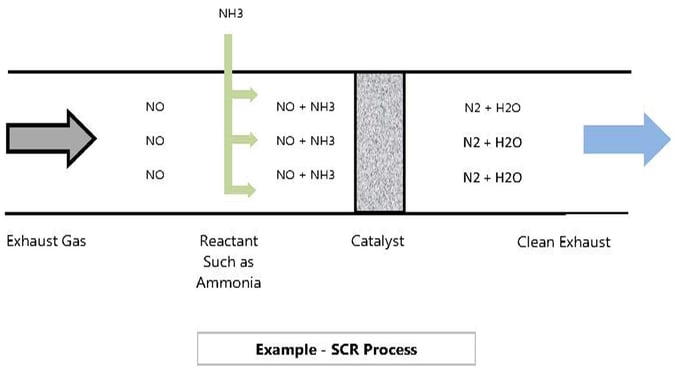
Selective Reduction uses oxygen from the NOx, not from the oxygen in the exhaust gas.
A traditional SCR catalyst is comprised of Vanadium Oxide and requires the gas stream to be heated to 400° F to 650° F. The Vanadium Oxide is evenly dispersed over a Titania Oxide extruded monolith.
Zeolites are also being used in different SCR processes where the exhaust gas may have certain contaminates such as sulfur, or when the exhaust is at very high temperatures that prohibit the use of Vanadia-Titania Catalyst. These products normally operate in the range of 500° F to 1,100° F.
In each case, a reactant or reductant is required to mix thoroughly with the exhaust gas before it reaches the catalyst bed. The corresponding reactions are summarized below:
4 NH3 + 4 NO + O2 = 4 N2 + 6 H2O
2 NH3 + NO + NO2 + O2 = 4 N2 + 6 H2O
8 NH3 + 6 NO2 + O2 = 7 N2 + 12 H2O
Depending on the exact constituents of the exhaust gas, there may be a number of side reactions that must be understood. Some examples side reactions may include:
4 NH3 + 5 O2 = 4 NO + 6 H2O
2 SO2 + O2 = 2 SO3
2 NH3 + SO3 + H2O = (NH4)2SO4
A Reductant or Other Compound That Is Mixed With the NOx.
As indicated in the steps above, a reducing agent must be used in the SCR process. The reductant provides a source of hydrogen to be used towards the goal of creating water as the byproduct compound. Other reductants maybe used, but ammonia is most commonly used, due to its availably and ease of decomposition.
There are several methods of using ammonia as a reducing agent. Each method has advantages and disadvantages and should be thoroughly considered when planning any NOx abatement project.
Anhydrous Ammonia is very toxic and difficult to safely handle, but it does not require auxiliary treatment in order to work effectively in the SCR process.
Aqueous Ammonia is much safer to store and deliver to a NOx abatement system. It does require vaporization by such means as pre-heating or fluidized injection.
Urea is the safest of all these reductants, but it requires thermal decomposition to ammonia in order to be an effective reductant. This means that higher capital and operating costs are necessary to treat NOx using Urea as the reductant.
The SCR process can typically obtain removal efficiencies from 90 to 95%. Higher efficiencies may be obtained in hybrid systems or through the use of an additional catalyst. Other system design considerations may include:
Exhaust Analysis – Careful analysis and consideration of the target exhaust gas must be conducted. Since SCR is a catalytic process, all the challenges of using catalysts apply in the SCR process. Consideration of particulates and catalyst poisons should be reviewed with any equipment vendor. There may be other pollutants such as Carbon Monoxide (CO), Volatile Organic Compounds (VOC) or Hazardous Air Pollutants (HAP) that require treatment. In these cases, a hybrid system maybe be required. You can learn more about catalyst design basics by visiting this link.
Ammonia Slip – this refers to the amount of residual or excess ammonia left over in the treated exhaust gas. Ammonia as a pollutant must be considered and may be part of any air permit a SCR process will have to address. To learn more about the EPA’s move to target ammonia, visit this link to our E-book on the subject. Excess ammonia slip may be controlled by adding a second catalyst bed with the purpose of “polishing” the exhaust of residual ammonia. A standard precious metal catalyst can be an effective means of eliminating ammonia, but careful design must consider that as ammonia is converted, the resulting byproduct is NOx. Newer generation ammonia catalysts are offered that are able to selectively convert ammonia with minimal NOx emissions.
Continuous Emission Monitoring (CEM) – Certain processes that have high NOx emissions may be required by the EPA to utilize Continuous Emission Monitoring Equipment (CEMs). Certain processes that have fluctuations in NOx concentration can benefit from the use of CEMs to precisely control the flow of reactant. CEMs are analytical instruments that provide constant measurement of NOx and/or ammonia in the exhaust stack.
For additional information or if you have any questions about SCR and NOx abatement, call us at 847.550.4339 or










