Many pollutants, both natural and synthetic, are gaseous in nature and require specific technology to effectively remove the pollutant from an exhaust or process gas stream. Gaseous industrial pollutants can include:
- Acid Gases (hydrochloric acid, sulfuric acid, hydrogen sulfide, and many others)
- Inorganic Gases (Sulfur Oxides, Nitrogen Oxides, Ammonia, etc.)
- Organic Gases (Ethylene, Benzene, Ethanol, and many other volatile organic compounds [VOCs] or hazardous air pollutants [HAPs])
There are several technologies available that can provide control (removal) of gaseous pollutants. This blog will provide you with a high level description and review of adsorption as an air pollution control technology for organic pollutants. The most common adsorbents used in industry are activated carbon, silica gel, activated alumina (alumina oxide), and zeolite.
Future blogs will examine other technologies that may provide an alternative to oxidation/incineration.
While CPI does not provide all of the available technologies, we do take our consultative application analysis seriously, and when a more appropriate technology may offer some advantage, we will present other treatment options to our clients.
If you missed our previous blog discussing Absorption, you can read it here.
ADSORPTION
Adsorption is a mass transfer process in which a porous solid comes in contact with a liquid or gaseous stream to selectively remove pollutants or contaminates by depositing (adsorbing) them onto the solid. The contaminates being removed are referred to as the adsorbate, and the solid doing the adsorbing is called the adsorbent.
Adsorption devices are commonly used in many industrial applications. In general, certain organic and inorganic compounds with a molecular weight greater than 45 are likely to be a good adsorbate. Adsorption is often used in applications where recovery of the adsorbate is desired or if the concentration of the adsorbate is very low and other types of pollution treatment would be cost prohibitive. Some common industrial operations in which organic emissions and odors can be effectively controlled by adsorption include dry-cleaning, degreasing, surface coating, rubber processing, gravure printing, and others. Adsorption can also be used to purify intake or circulating air streams and in fractionization of certain gases.
The adsorption process can be both a physical and chemical process:
Physical adsorption - The adsorbate molecules (the contaminate) adhere to the adsorbent materials in a physical bonding force referred to as “van der Waals forces”.
Chemical adsorption - a chemical bond is created between the adsorbate and adsorbent. This bond is referred to as “chemisorption.” Chemisorption usually occurs at elevated temperatures when energy is necessary to break chemical bonds. Chemisorption is essentially the same process as catalytic oxidation. More information about catalytic oxidation can be found here.
PHYSICAL ADSORPTION
The most common adsorbents used in industry are activated carbon, silica gel, activated alumina (alumina oxide), and zeolite. Some of the characteristics important to adsorbent selection are port size and distribution, particle size, chemical nature, surface area, and surface polarity. Polarity determines the type of adsorbate for which any particular adsorbent will have the best performance. Activated carbon is the most common non-polar adsorbent. Polar adsorbents have a great attraction to absorb moisture. Since most industrial exhaust streams contain moisture, the use of polar adsorbents is significantly limited for air pollution control systems.
The most efficient method for promoting adsorption is in a packed bed through which the polluted air stream being treated passes. As the stream passes through the bed, adsorption of the contaminates takes place, and the purified air exits the packed bed. An adsorbent has a fixed amount of contaminates that it can hold. The exact capacity is based on many factors and is referred to as the adsorption isotherm. One way to think about the adsorption capacity is to consider a common sponge holding onto water. In this example, water is the adsorbate and the sponge is the adsorbent. The sponge has many pores in which to adsorb the water. Once these pores are completely filled, the sponge cannot accept any more water. This is called breakthrough, where the adsorbent is completely saturated with adsorbate and is no longer effective.
Types of Adsorption Systems
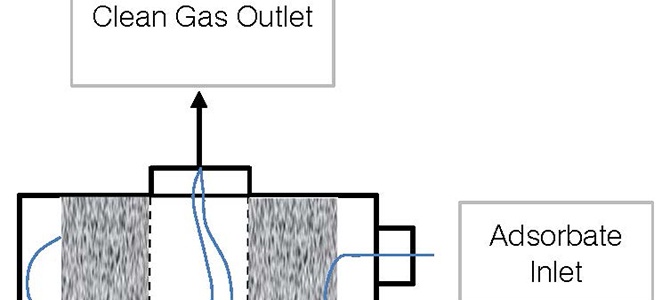
- Fixed bed (packed bed) - These are simple devices that maximize surface area to promote full and even use of the adsorbent. As shown in the below flow diagram, the contaminated gas enters the fixed bed vessel at the side. The adsorbent material (consider this to be activated carbon) is contained in a packed bed arrangement that allows for as much exposed surface area as possible. The polluted air enters the activated carbon packing and works towards the center, where there is an exhaust distributor. The exiting exhausted air is clean of pollutants or contaminants.
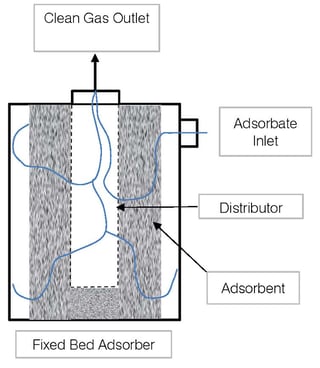
Once the adsorbent is fully saturated with adsorbate (leading to breakthrough), the system requires change-out of the spent materials with new materials. This is typically accomplished through a sub-contractor that specializes in adsorbent materials and service. The spent adsorbent will be thermally cleaned at off-site facilities specially designed for this purpose.
- Continuous systems are more complex and provide continuous operations without having to conduct manual change-out of adsorbents. These systems provide in-situ desorption of the adsorbates from the adsorbent. This can be accomplished with superheated or saturated steam. In these systems, the adsorbate can be condensed, collected, and re-used in the process. The process is exactly as described above, but there are two adsorbers used in the system. The gases are being adsorbed in one unit as the other unit is being desorbed with steam. The exhaust from the desorbed bed can be condensed for solvent reuse or other beneficial purpose.
A second type of continuous adsorption device is a Zeolite VOC Concentrator, or Rotary Concentrator. In these systems, a hydrophobic zeolite is designed in a monolithic rotor in which the contaminated air stream flows over a large percentage of the rotor. An integrated thermal oxidizer is used to provide desorption of the solvents from the zeolite and final destruction. Rotary Concentrators or Zeolite Adsorbers have wide acceptance in industrial air pollution control applications that have very high volumes and low concentrations.
The use of adsorption systems as an air pollution control or VOC abatement device has wide acceptance. These systems are particularly popular for odorous gas streams, where the offending odor is caused by very low concentrations of organic materials. Moreover, these systems can provide cost effective abatement for low concentration contaminates and may even present an ability to recover or re-use the adsorbate. However, there are a number of limitations that should be understood:
- In general, organic concentrations greater than just a few parts per million (ppm) may complete saturation of the adsorbate and thusly require frequent media change-out. Depending on the adsorbent material and the frequency of change-out, the annual cost of operation can greatly exceed that of other treatment devices.
- Adsorbers are sensitive to particulates (as mist or solids) and may create a plugging event. Pre-treatment of mists and solids maybe required to extend the adsorbent life and present safe conditions for operation.
- VOCs with low vapor pressure tend to condense very easily. Condensations can adhere and accumulate on the adsorbent surface and negatively affect performance.
- Humidity can temporarily degrade performance. In general, if the water vapor is around 2.5 volume percent or greater, careful analysis should be conducted.
- The polluted air stream should be relatively low in temperature. A good rule of thumb is less than 100°F for most adsorbents.
- Some adsorbents may pose safety concerns due to combustible dust or reactivity with oxygenated organics that can create an exothermic condition.
- In some EPA regulated regions, the use of VOC analyzers may be required to insure performance (making sure that the VOCs are being adsorbed onto effective media). This equipment adds to the cost and complexity of the system and may not give adequate advanced notice of lower performance.
Adsorption is just one of the technologies used to remove gaseous pollutants. As mentioned, there are several other technologies that provide air pollution control as well. Please stay tuned as we continue to cover the following topics in upcoming blogs:
In the meantime, If you have any questions, please feel free to contact us.